Science
Source: NHLBI
Cardiovascular
Fetal growth, neonatal birth weights and survival are all directly related to major vascular adaptations in normal pregnancies. The lab's work demonstrates substantial vascular endothelial programming in pregnancy, adaptations to blood pressure, and uterine arterial vasodilation to accommodate the blood flow and nutrient requirement of the growing fetus.
Source: NHLBI
Environment Health and Pulmonary Toxicology
Our research utilizes validation methods including ultra-high-frequency ultrasonography, flexiVent, microspheres, RNA-SEQ, high throughput immunoassays, pressure myography, and chromatin immunoprecipitation to assess environmental consequences of secondhand vaping of e-cigarette and to phenotype both maternal and developmental physiologic responses
Source: NIH
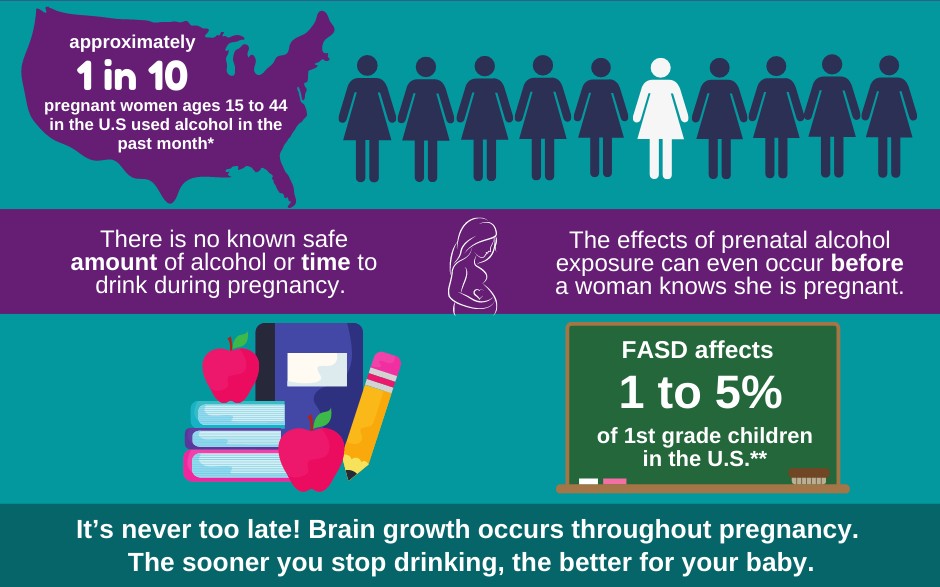
Child health and alcohol teratology
Although alcohol produces a range of growth and neurodevelopmental deficits, we are only beginning to understand the specific molecular targets of alcohol (ethanol). Our lab employs a multi-tiered holistic approach (whole animal physiologic and cellular/molecular) and utilizes powerful validation methods including ultra-high frequency ultrasonography, microspheres, RNA-seq, pressure myography, HPLC, electrophysiology, and 3D dendrite morphology assays to assess alcohol teratogenesis.
Source: Reprod Toxicol. 2018.76: 8492
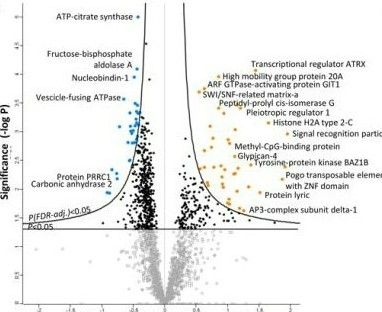
High throughput approaches
We have utilized mass spectrometric technologies to identify protein targets of alcohol in fetal brain regions, the placenta, and the mother's uterine artery in a rat model. Identifying etiological cues from these explorations, and clustering these cues into patterns, we developed a model of the uterine artery's adaptations in FASD, and directly related these adaptations to the cardinal outcome of fetal growth restriction.
We have utilized several novel complimentary quantitative mass spectrometric approaches including non-labeled (label-free) nano LC MS/MS, gel-based 2-D DIGE MALDI TOF/TOF, and label-based methods like iTRAQ nano LC MS/MS to study differential protein signatures during pregnancy and also validated them.
Source: NIH
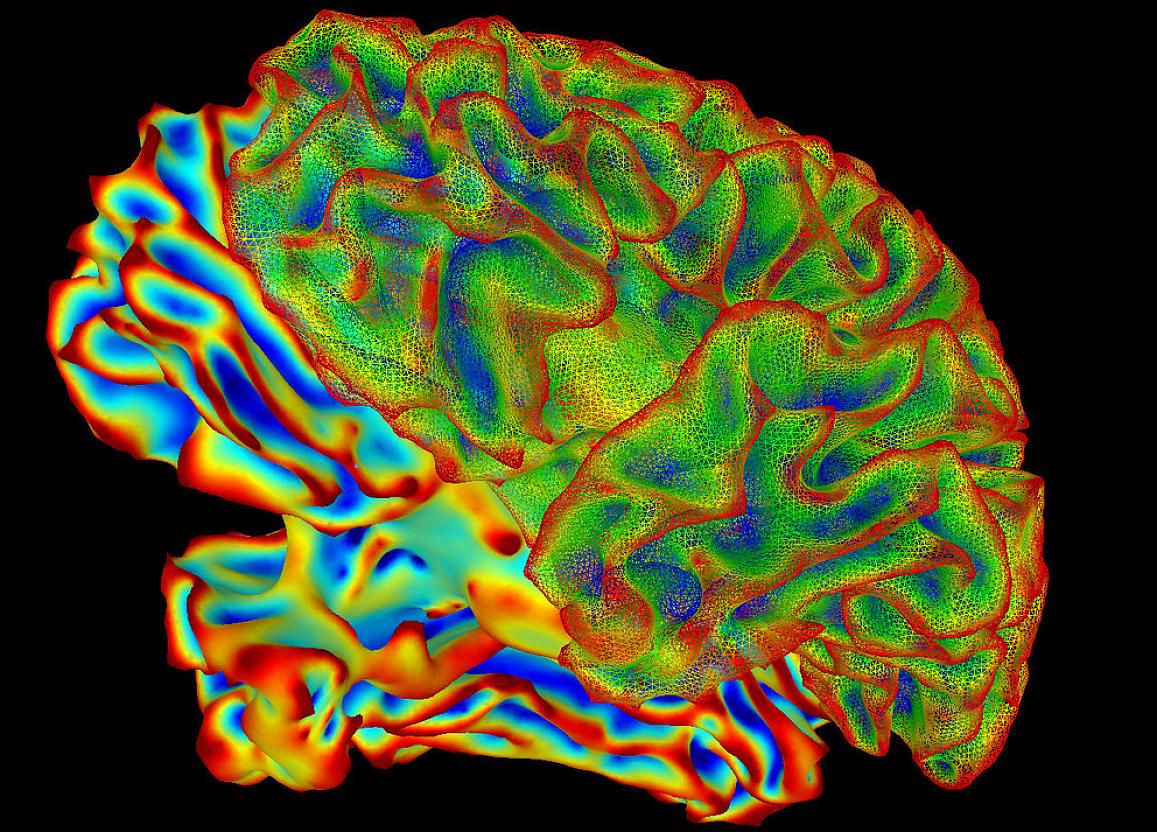
Neuroscience
The laboratory has moved from merely cataloging alcohol-induced fetal deficits to identifying factors contributing to the etiology of FASD. The lab has developed methods to identify novel etiological factors affecting FASD outcomes. This was aided by exploiting the similarities in molecular targets of alcohol between the mother's uterine blood vessels and the fetal brain arteries. Since much of the FASD field focusses on neurobiology and behavior, our physiologic approach to characterize fetal brain hemodynamics is both novel and valuable. Employing a whole animal physiologic and cellular/molecular approach, the lab envisions assessing the effects of pharmacological and/or nutraceutical agents on prevention of FASD.